ADI Proteome Microarray Technology:
Antigen Discovery Inc. has developed a high-throughput technology to translate any genome to proteins printed as individual spots on a microarray. In the last twelve years, we have built a large collection of expressible open reading frame (ORF) libraries from over 50 medically-important infectious microorganisms and we are constantly expanding our collection. Below, we outline the four steps in our platform technology that direct your research from genome to antibody binding data in a matter of a few weeks.
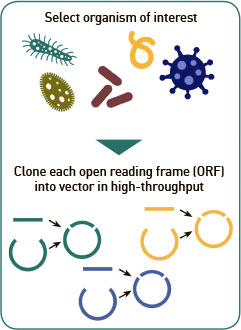
Genome library: ORF expression clone libraries can be constructed from any genome sequence and corresponding source of genomic DNA using our high-throughput polymerase chain reaction (“PCR”)/recombination cloning method. PCR-amplified ORFs of the target organism are cloned into our plasmid expression vector via a high-throughput homologous recombination method. This step can take a few weeks up to a few months depending on the size of the proteome and quality of the DNA template. Follow the link to see our large collection of currently available proteomes here (Link to Proteome library). New proteome libraries are constantly being added and we will work with you to construct any new proteome you need. Find out more in our Resources section.
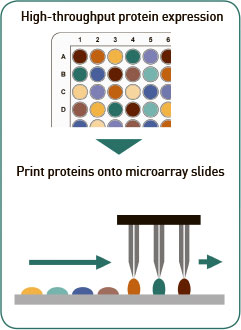
Protein microarray: Proteins encoded by the cloned ORF plasmids are expressed using a cell free in vitro transcription/ translation (“IVTT”) system. Each protein is expressed and printed individually onto custom nitrocellulose microarray slides. Every batch of printed protein microarray slides is quality control tested by automated scanning to inspect for spot deposition and morphology; and for protein expression using antibodies against the C-terminal polyHis and N-terminal polyHA tags.
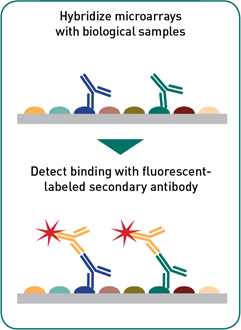
Sample screening: With as little as 2-5 μL of serum or other antibody-containing biological specimen the complete or partial proteome can be interrogated on a single slide for antibody binding. Isotype-specific bound antibodies are detected and using a fluorescently labeled secondary antibody. Our microarrays have be probed with a variety of human specimens e.g. serum, plasma, antibodies in lymphocyte supernatant (ALS), fecal samples, breast milk; and with animal samples (domestic and farm animals) where secondary antibodies are available (e.g. mouse, rat, rabbit, monkey, cattle, etc.).
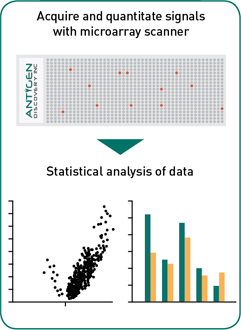
Data analysis: Using a fluorescence microarray scanner, data from protein microarrays is acquired, and checked for quality. Working with your team, our dedicated team of bioinformatics analysts run the data through a proprietary statistical analysis pipeline to construct heatmaps, develop univariate and multivariate statistical models for group comparisons to help analyze and interpret results and generate publication-quality figures to visualize the data.
*time frames mentioned are an estimate, actual length varies for each project. With your custom quote you will also receive an expected time line for your project.
ADI High-Throughput T Cell Screening:
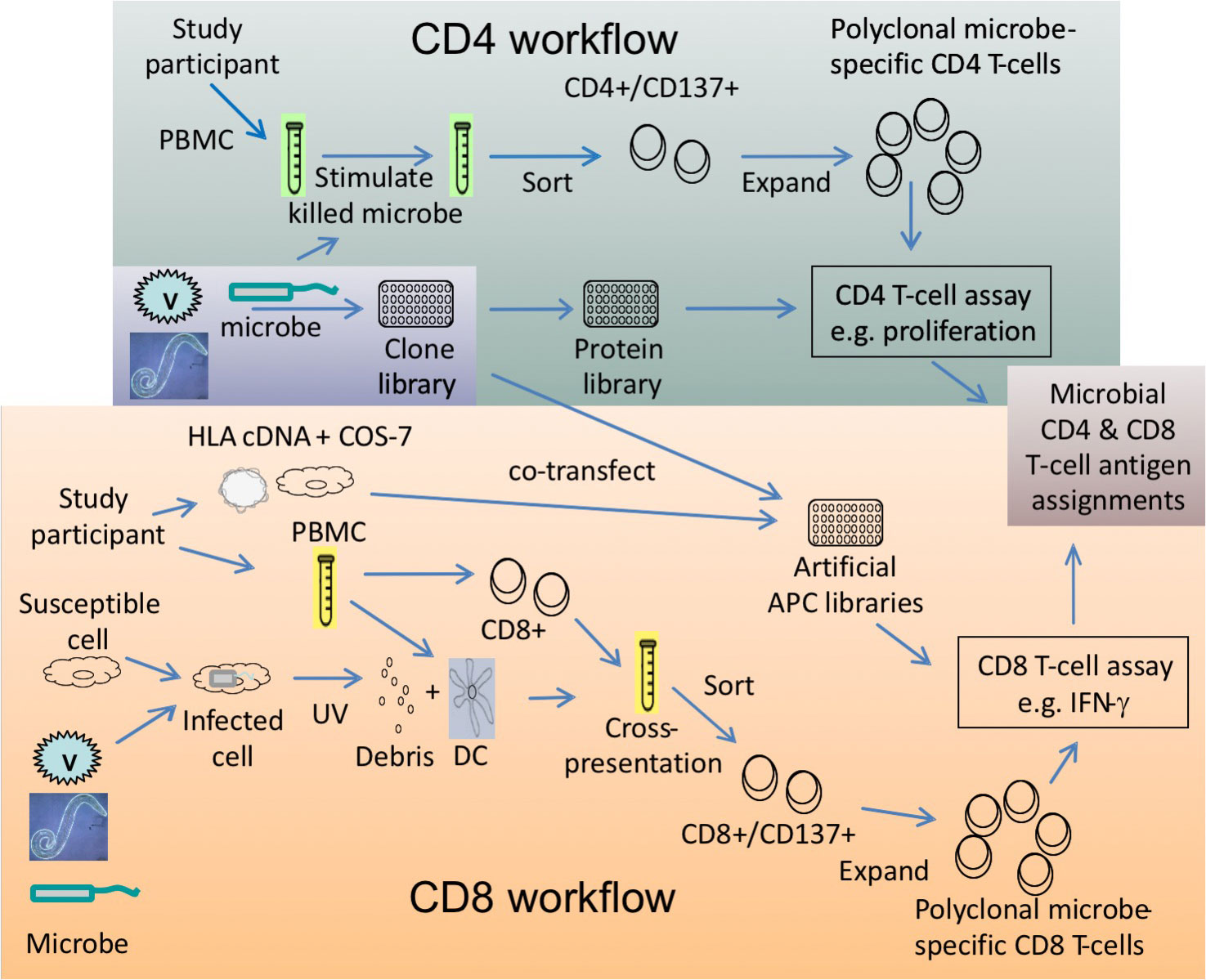
Antigen Discovery Inc., in collaboration with David Koelle’s laboratory Laboratory at the University of Washington in Seattle, has developed a high-throughput screening methodology for identification of antigen-specific CD4 and CD8 T cell specificities in cellular samples. This technology has been demonstrated for viral and bacterial proteomes, with work underway on the first proteome of a parasitic disease. Workflows have been developed for the expansion of pathogen-specific CD4 or CD8 T cells expressing CD137, which can then be used in screens against pathogen protein libraries to identify those that specifically activate T cells (e.g. proliferation or cytokine secretion). The antigens discovered can be used in downstream applications such as single-cell phenotyping with multiparameter flow cytometry and epitope mapping. Find out more in our Resources section.
Given sufficient lymphocytes in a cellular sample (e.g. peripheral blood mononuclear cells, or PBMC), killed microbes or microbial material is used to stimulate PBMC and sort the CD4+CD137+ T cells, which are then expanded to >108 pathogen-specific enriched T cells. Expanded T cells are screened in stimulations with pathogen proteins in the presence of autologous PBMC as antigen-presenting cells (APC). A read-out assay such as 3H-thymidine incorporation or cytokine secretion is used to identify the targets of antigen-specific responses.
Given sufficient lymphocytes in a cellular sample, PBMC can be stimulated to activate pathogen-specific CD8 T cells. The stimulation conditions differ from the CD4 workflow, in that the stimulus is UV irradiated cells infected with the target microbes or UV-irradiated cellular microbes, which are added with autologous PBMC-derived dendritic cells as APC for cross-presentation to CD8 T cells in PBMC. CD8+CD137+ T cells are sorted and expanded to >108 pathogen-specific enriched T cells. Expanded T cells are screened in stimulations with pathogen proteins that are cross-presented to CD8 T cells through an artificial APC library generated from matched HLA and pathogen clones co-transfected into COS-7 cells. A read-out assay such as cytokine secretion is used to identify the targets of antigen-specific responses.
Applications
Our high throughput technology enables the identification of proteins useful for the development of a wide range of therapeutics, vaccines, and diagnostics. It expedites biomarker discovery by measuring responses to thousands of proteins in a single assay.
How we are unique:
- First company to leverage genomics era information into proteomics for biomarker discovery
- Proprietary platform screens the entire proteome and identifies important antigens and biomarkers in a single assay
- We have generated over 60,000 ORF clones from >50 medically relevant disease-causing organisms
- Full service provider of project execution, from study design to data reports, IP filing and publication
- Leader in statistical analysis of protein microarray technology
- Over 120 peer-reviewed scientific publications using our technology and expertise
- Site reference laboratory for microarray equipment manufacturers, consumables suppliers and end users
- Collaborative company culture with shared interests in academia and industry
ADI’s novel approach eases the R&D bottleneck and drastically decreases the time required to perform proteome-wide screening by eliminating the time consuming steps involved in traditional cloning, protein expression and screening methodologies. This means that proteomic screening and antigen identification can be done in weeks instead of years. Our platform is robust and flexible, easily fitting any needs of your studies to identify diagnostic biomarkers, vaccine candidates and therapeutic antibodies. Our microarray platform facilitates validation of monoclonal antibody targets and is a useful tool in immunoepidemiology studies (e.g. population-level antibody profiling).
Vaccines
Our immunoprofiling platform is among the most efficient technologies available for rapid discovery of new vaccine candidate antigens for pre-clinical and clinical development. Applications of the technology for vaccines include:
- Discovery of candidate antigens for subunit vaccines through analysis of correlates of protection
- Immunogenicity studies for optimization of vaccine formulations and dosing regimens
- Sub-typing of immune responses (e.g. antibody isotype and subclass)
- Quantification of adjuvant effects on the immune response after vaccination
Clinical Trial Tools
Clinical trials are often affected by factors that can alter the progress, outcomes, or statistical power of the study. Our technology can be included in the protocol of clinical trials for multiple applications:
- Screening volunteers before a trial begins for biomarkers of pre-existing immunity to the intervention or primary outcome (e.g. pathogen challenge)
- Measure immune responses that correlate with the treatment protocol and observed efficacy, leading to the development of companion diagnostic tests
- Identify biomarkers of infection, disease severity and resolution or other outcomes, which may be used as primary outcomes in lieu of higher risk procedures (e.g. prolonging time to curative treatment)
Antibody Target Validation
ADI’s proteome microarray technology can provide valuable information on the identification and quality of commercial or newly derived monoclonal antibodies. Monoclonal antibodies that bind specifically to the intended target can be identified, or the unknown protein target of a monoclonal can be identified. The literature is full of examples of monoclonals that unexpectedly bind to multiple different proteins or to the wrong protein which invalidates the results. Find out more in our Resources section.
Therapeutic Antibodies
New monoclonal antibody therapies for the prevention (prophylactic) or resolution (therapeutic) of disease are becoming available, but the target antigens of these antibodies are often unknown. ADI’s proteome microarray technology can provide valuable information on the identification and quality of new monoclonal antibodies:
- Discovery of candidate antigens for monoclonal antibody development through analysis of correlates of protection
- Therapeutic antibodies derived from plasmablasts, hybridomas or synthesized as recombinant antibodies from variable region gene sequences can be probed on pathogen proteome microarrays to identify the target protein
- Polyclonal antibodies used to resolve infections can also be probed on proteome microarrays to identify the multiple antibody targets that resolve disease
- On-target/off-target predictor of immune related complications (e.g. cross-reactive autoantibodies)
Diagnostic Applications
Antibody-based detection of infectious diseases is an ideal and cost-effective platform for diagnostic testing. Our proteome microarray technology facilitates discovery of proteins targeted by antibodies that provide both sensitive and specific detection of infection and active disease. With well-characterized samples, we provide a clear pathway from discovery to development of a new diagnostic test.
Biomarker Discovery
At its core, ADI’s immunoprofiling technology is geared toward biomarker discovery. The usefulness of biomarkers extends beyond correlates of protection and diagnostics. Biomarkers serve diverse functions, including disease transmission monitoring, recency of exposure, markers of “vaccine take”, and disease predisposition, susceptibility and resistance. Our proteome screening tools are flexible and can easily address most study questions.